Dear Valued Customers:
ICU Medical, Inc. is issuing this Urgent Medical Device Recall letter to notify you of a potential for leaks to occur with the Spinning Spiros™ Male Luer in certain lots. The attached urgent medical device recall letter details the issue and the required steps for you to perform.
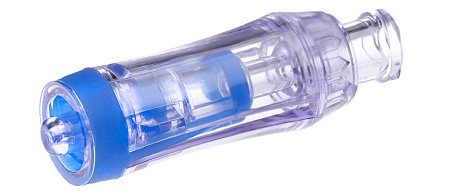
Issue:
ICU Medical has identified the potential for certain lots of the Spinning Spiros to exhibit leaks due to a molding defect. This information pertains to the spinning version of the Spiros only. The non-spinning version is not affected by this communication.
Potential Risk:
Fluid leakage may potentially cause delay of infusion, contamination of the fluid path, exposure to hazardous medications, blood loss, exposure to patient blood, or fluid path air-in-line. ICU Medical has received reports of leaks potentially related to this issue and has not received reports of permanent injury or death.
Affected Product:
Affected products were distributed in the United States between January 2020 and July 2020. The affected item and lot numbers are provided in Table 1 of the attached recall letter.
If you have any questions regarding this recall, please contact your IMS Sales Representative or call Customer Service at 800-755-3800.